WHAT IS ANTIGEN TESTING. Significant Testing Capacity.
Laboratory Testing For Coronavirus Disease 2019 Covid 19 In Suspected Human Cases Interim Guidance 2 March 2020
If the test returns positive for COVID-19 you will usually receive the result within 12 days.

Covid-19 laboratory test report. 13 Main Street Cleve SA 5640. Tests for the etiological agent identify. However it is possible for this test to give a negative result that is incorrect false negative in some people with COVID-19.
Our lab serology blood tests running on ARCHITECT and Alinity i systems are used for the detection of antibodies IgG and IgM and identify if a person was previously infected with the virus that causes COVID-19. PCR tests are mainly for people who have symptoms. Aegis COVID-19 Testing.
If you have not received your result within these timeframes you need to contact the GP who ordered your test. You may kindly contact the testing lab to upload the results immediately in the ICMR portal wwwicmrgovin. Laboratory testing for COVID-19 in suspected human cases.
Youll usually get a text or email with your result when its ready. Aegis TAT is measured from the time samples are logged into our laboratory tracking system until test reports are released. Theyre sent to a lab to be checked.
Open 200 pm to 400 pm Mondays Wednesdays and Fridays. If you use the NHS COVID-19 app you may also get your result in the app. The guidance for electronic submission of COVID-19 testing results has been updated.
An antibody is a protein that the body produces in the late stages of infection. It does not include transit time to lab.
This means that you could possibly still have COVID-19 even though the test is negative. You will also receive the result via SMS to your registered. Use this service to report your result to the NHS after using a rapid lateral flow test kit to check if youre infectious with coronavirus COVID-19.
Aegis works with all of its collection partners to maximize the use of overnight shipping to its lab after collection. Active capacity of 25000 COVID-19 tests. Point-of-care tests can be used outside the laboratory setting by a health practitioner or trained staff under their supervision to test a person for COVID-19.
Antigen tests detect proteins of the SARS-CoV-2 virus. If the result is positive a unique patient code eg. The role of rapid disposable tests for antigen detection for COVID-19 needs to be evaluated and is not currently recommended for clinical diagnosis.
This ensures a suitable health practitioner or trained person under their supervision is available to ensure an adequate sample is collected correct interpretation of results and provide immediate clinical. Once the Lab uploads the result in the ICMR portal the result will be available on this page within an hour. If this is the case your.
Download Corona virus covid 19 tube test medical laboratory. The University of New Hampshire posts results from its ongoing regular COVID-19 testing of faculty staff and students on all three of its campuses Durham Manchester Concord and vaccination information every weekday excluding holidays. PCR test result.
We have a huge range of Illustrations products available. Searches in PubMed and Google Scholar for articles made available in 2020 using the terms diagnosis OR diagnostic OR diagnostic tests OR tests AND COVID-19 OR SARS-CoV-2 in the title. If negative you will usually receive the result in 24 days.
Coronavirus disease 19 COVID-19 is the greatest pandemic in modern history. Shipping delays and weekend shipping. The aim of this study was to investigate differences and prognostic.
Our seasoned staff of pathologists scientists technologists and support personnel are committed to delivering high-quality test results within 24-48 hours of receipt at our facility. 139900 will be performed at the Center for Esoteric Testing in Burlington North Carolina or. The COVID-19 RT-PCR Test Labcorp Laboratory Test Number.
COVID-19 Test Home Collection Kit when directly ordered by an HCP. For COVID-19 a negative test result for a sample collected while a person has symptoms usually means that COVID-19 did not cause your recent illness. The health and safety of our community remain our top priorities and this data is reviewed.
BU number for Bangalore will be created for you. By appointment no referral required. Effective May 12 2021 laboratories that process COVID-19 test results are no longer required to report daily aggregate testing data.
Cleve COVID-19 testing service. COVID-19 Lab Testing Dashboard. Coober Pedy Community Health COVID.
Search COVID 19 Sample Test report. The Coronavirus Aid Relief and Economic Security CARES Act and its June 4 implementation guidance external icon require every CLIA certified COVID-19 testing site to report every diagnostic and screening test result both positive and negative results performed to detect SARS-CoV-2 or to diagnose a possible case of COVID-19 eg molecular antigen antibody to. Phone 1300 334 222.
This was a non-systematic review of the literature on the laboratory diagnosis of COVID-19. A rapid lateral flow test is a coronavirus. The Ohio Department of Health ODH has rescinded a May 13 2020 order to Ohio laboratories to report the aggregate results of COVID-19 tests.
The COVID-19 RT-PCR Test can also be used to test pooled samples using a matrix pooling strategy ie group pooling strategy. Laboratory test alterations have been described in COVID-19 patients but differences with other pneumonias have been poorly investigated to date especially in Caucasian populations. Serological assays will play an important role in research and surveillance but are not currently recommended for case detection and are not included in this document.
Northwest Laboratory is a national clinical laboratory offering high-volume rapid turnaround COVID-19 testing services. Depending on where you were tested a negative result may be sent to you by SMS or a phone call from your doctor. Most people get their result the next day but it may take up to 3 days.

What Is The Diagnostic Accuracy Of Antibody Tests For The Detection Of Infection With The Covid 19 Virus Cochrane

Sample Reports Diagnostic Solutions Laboratory

Coronavirus Disease Covid 19 Situation Report 154 22 June 2020 World Reliefweb
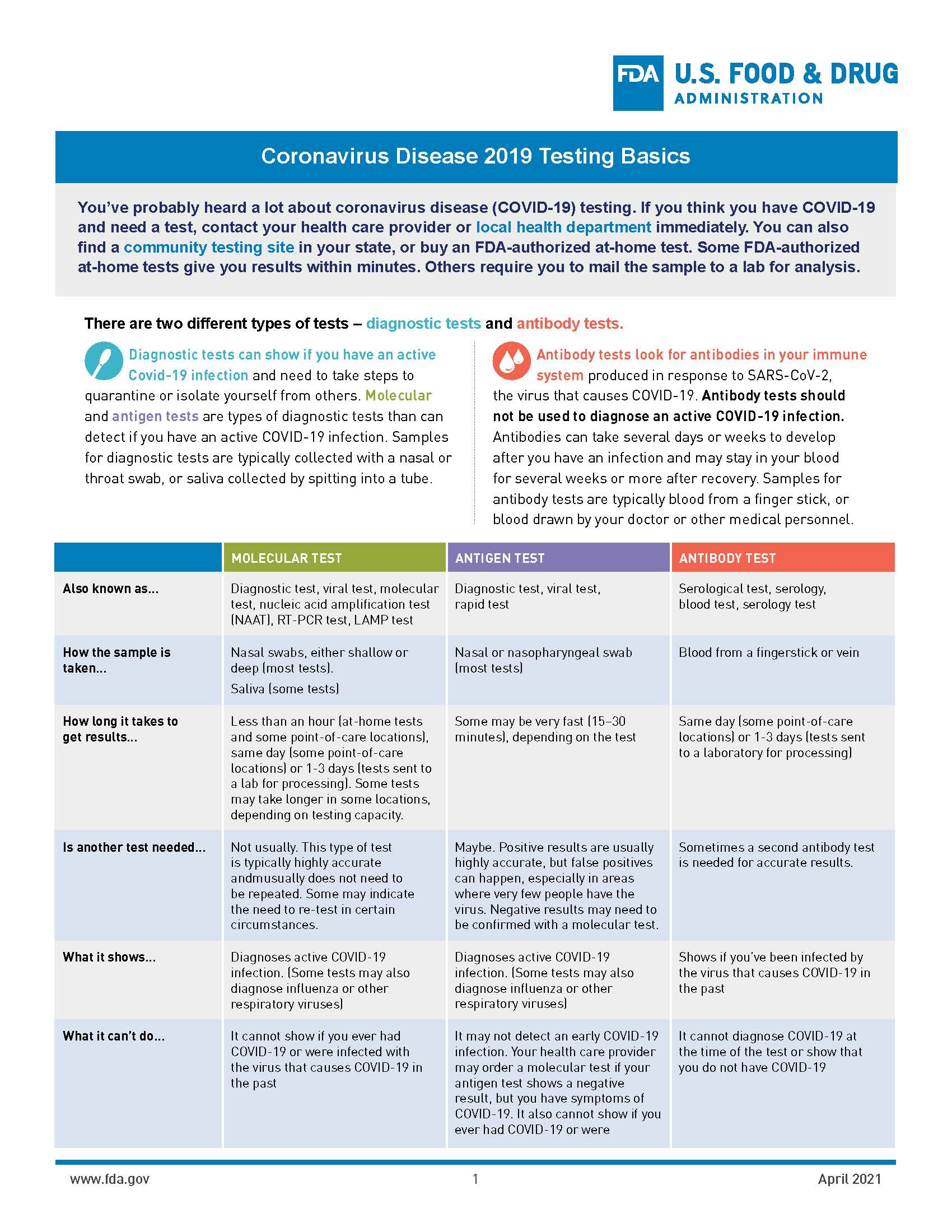
Coronavirus Disease 2019 Testing Basics Fda

Covid 19 Pcr Test For Travel Clinical Labs

Tidak ada komentar:
Posting Komentar