This FDA-authorized test helps see if youve developed an immune response and may not be at immediate risk of COVID-19 reinfection. Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Coronavirus disease COVID-19.

This Antibody Test Could Offer A Clearer Picture Of How The Body Responds To Covid 19 Pbs Newshour
Component Test Code Component Chart Name LOINC.

Cpt code for covid 19 antibody blood test. Antigen testing is designed to be quicker and simpler than other tests for the coronavirus and can be conducted at the point of care. View testing statistics. 15 2021 UnitedHealthcare will reimburse the appropriate COVID-19 monoclonal antibody treatment codes for in-network urgent care facilities.
Things to know Medicare also covers COVID-19 tests COVID-19 monoclonal antibody treatments and COVID-19 vaccines. SARS-CoV-2 antibody often referred to as serology tests look for antibodies in a sample to determine if an individual has had a past infection with the virus that causes COVID-19. Food and Drug Administration FDA.
Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. Covid 19 Blood Test Cpt Code - Safely Save Your money. Monoclonal antibody treatment Q0240 M0240 Q0243 M0243 Q0244 Q0245 M0245 Q0247 M0247 Effective Oct.
The Pfizer booster dose was effective on Sept. New information that I find in the course of my daily studies will appear on this site on a weekly basis. COVID-19 IgG Index by CIA.
A table of quantitative anti-spike levels for otherwise healthy recently vaccinated individuals by week of vaccination to aid in interpretation of test results is available in Table 3 in this pre-print. Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Coronavirus disease COVID-19 amplified probe technique Development review and approval of the new CPT code involved broad input from practicing physicians commercial laboratories the Centers for Disease Control and Prevention CDC and other experts. A COVID-19 vaccine code claim for the same patient UnitedHealthcare will deny the vaccine code.
The American Medical Association AMA today announced two new CPT codes to report when patients receive blood tests that detect COVID-19 antibodies. HIV-1 IgG WB CSF. New information that I find in the course of my daily studies will appear on this site on a.
Access COVID-19 Test Results. On May 19 2021 the FDA issued a safety communication reiterating that antibody testing should not be used to evaluate a persons level of immunity or protection from COVID-19 at any time and especially after the person received a COVID-19 vaccination4 Currently authorized SARS-CoV-2 antibody tests including the SARS-CoV-2 Semi-Quantitative Total Antibody assay 164090 have not. Test has been issued Emergency Use Authorization by the US Food and Drug Administration.
Im very excited to be able to share with you what I have learned over the past many years of study and hands-on practice with thousands of patients. The new codes are intended for use as the industry standard for accurate reporting and tracking of. The FDA has yet to authorize the use of neutralization tests for the novel coronavirus according to the CDC.
ENA Panel Erythropoietin ESR Estradiol EstrogenProgesterone Receptors Estrogens Ethanol Factor V Leiden Fecal Fat Fecal Occult Blood Test Ferritin fFN Fibrinogen FIP1L1-PDGFRA First Trimester Screen Flu Tests Folate Free Light Chains Fructosamine FSH Fungal Tests G6PD Gastrin Genetic. A special edition of CPT Assistant the official source for CPT coding guidance offers advice about the new codes for SARS-CoV-2 serologic laboratory testing. Code 86328 should be used.
Serologic results should not be used as the sole basis to diagnosis or exclude recent SARS-CoV-2 infection. This test is recommended in individuals at least 10 days post symptom onset or. 86769 Antibody.
If a specimen is collected too early prior to sero-conversion the test will yield a negative result. Qualitative detection of IgM antibodies to SARS-CoV-2 the virus that causes COVID-19 to help identify individuals who have been exposed to the virus. Clinical utility IgG antibodies to SARS-CoV-2 are detected in the majority of individuals approximately two weeks after the onset of COVID-19 symptoms.
The COVID-19 IgGIgM Rapid Test Cassette Whole BloodSerumPlasma is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2. AMA releases two CPT codes for COVID-19 antibody testing Apr 13 2020 - 0435 PM The American Medical Association Friday released two Current Procedural Terminology codes 86328 and 86769 for reporting antibody testing for the novel coronavirus and revised its CPT code for SARS-CoV-2 nucleic acid tests 86318. On May 19 2021 the FDA issued a safety communication reiterating that antibody testing should not be used to evaluate a persons level of immunity or protection from COVID-19 at any time and especially after the person received a COVID-19 vaccination4 Currently authorized SARS-CoV-2 antibody tests including the SARS-CoV-2 Semi-Quantitative Total Antibody assay 164090 have not.
Order Code Update Archive. The updates were approved April 10 2020 during a special meeting of the CPT Editorial Panel. Ratings 78 December 2021 News.
Covid 19 Fingerstick Antibody Test Cpt Code - Safely Save Your money. Im very excited to be able to share with you what I have learned over the past many years of study and hands-on practice with thousands of patients. New CPT code for SARS-CoV-2 COVID-19 antigen testing.
CPT coding is the. COVID-19 IgG by CIA Interpretation. The Moderna COVID-19 vaccine and immunization administration booster doses are effective as of Oct.
Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Coronavirus disease COVID-19. Ratings 78 December 2021 News. As with all the CPT codes related to COVID-19 vaccines the effective dates coincide with the emergency use authorization issued by US.
87635 Infectious agent detection by nucleic acid DNA or RNA. In contrast codes 86408 and 86409 are used to report tests that determine if the antibodies present can block the COVID-19 virus infection the organization explained. Turnaround time represents the number of business days from the date of specimen pickup to result release.

Ama Releases Two Cpt Codes For Covid 19 Antibody Testing Aha News

After A Covid 19 Diagnosis An Antibody Test Offered Me A Little Comfort

What Can Covid 19 Antibody Tests Really Tell Us Path

Covid 19 Testing Infographics British Society For Immunology
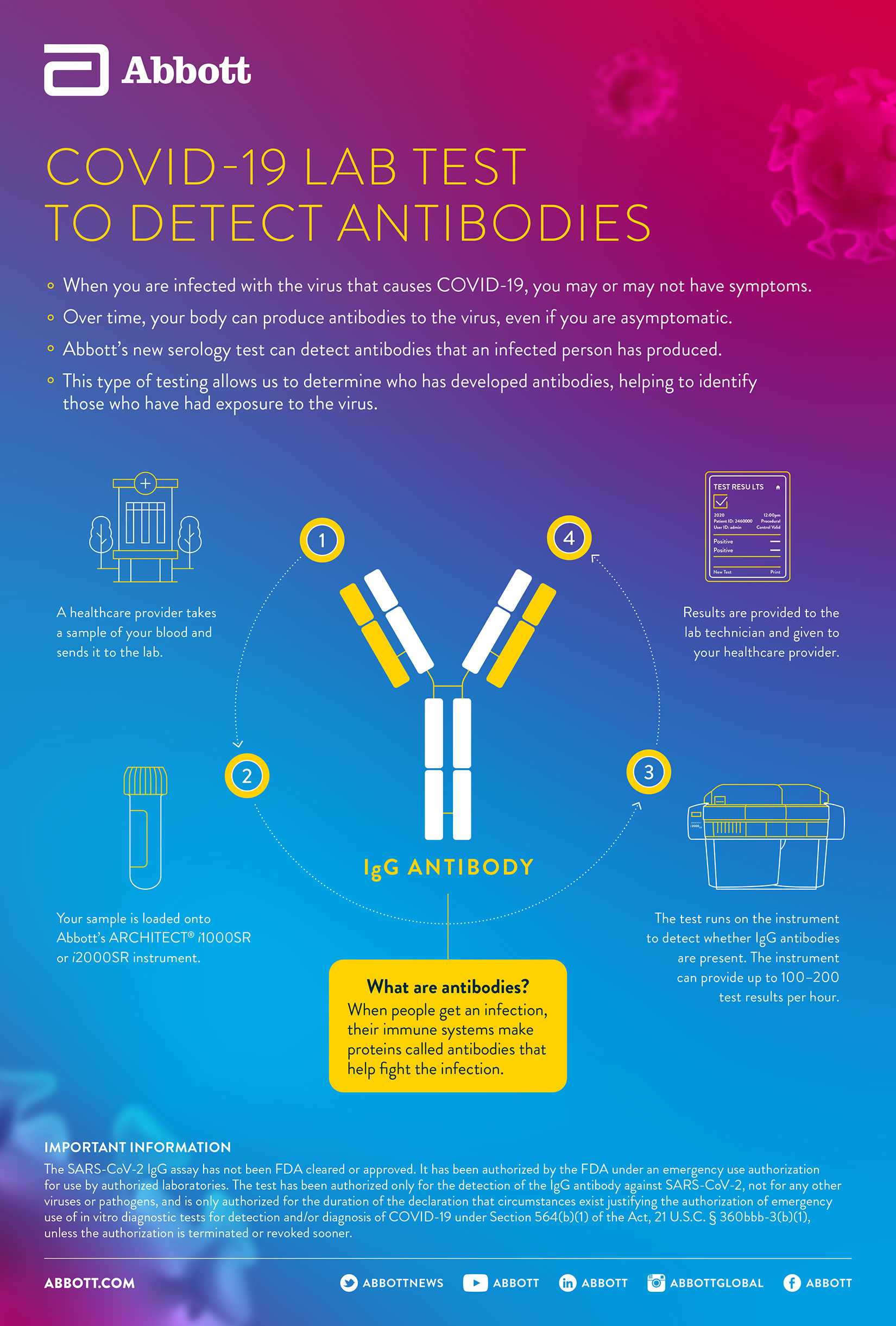
How Does Covid 19 Antibody Testing Work Abbott Newsroom

Tidak ada komentar:
Posting Komentar