The results were summarized below. There was no association between the sensitivities of the saliva S1 Fig or of nasopharyngeal swab S2 Fig estimated in each study and the prevalence of the virus in the same study.

What Is The Covid 19 Salivadirect Test
Auktionskaj 2 1sal 6700.

Covid-19 rt-pcr saliva test. However given the novel. Once the sample is collected the viruss RNA must be extracted before it can be detected by sensitive PCR-based methods. Patients who were suspected of COVID-19.
Omicron COVID-19 Variant. See Saliva Tests. At Clinical Labs we use the reverse transcriptase RT polymerase chain reaction PCR method which is a type of nucleic acid testing for our COVID-19 PCR test.
One Step Immunoassay Exdia COVID-19 Ag Precision Biosensor Daejeon Korea and Standard Q COVID-19 Rapid Antigen Test Roche-Switzerland. Primers attach to the. COVID-19 Assays CoviDetect Multiplex Assay.
First the PCR is converted from single-stranded RNA to double-stranded DNA in a process called reverse transcription. Is a saliva-based PCR test accepted. Are Rapid Antigen Tests RATs accepted.
SARS-CoV-2 is the etiologic agent of coronavirus disease 2019 COVID-19 and is mainly detected by RT-PCR methods from upper respiratory specimens as recommended by the World Health Organization. SARS-CoV-2 saliva testing is a useful tool for Covid-19 diagnosis J Virol Methods. Multiplex Assay CoviDetect Variants.
Other ways that one can be infected is by touching a surface or. A total of 58 paired NP-saliva specimens were collected. The PCR test looks for the presence of the COVID-19 virus by detecting its genetic material RNA through a technique called reverse transcriptase-polymerase chain reaction RT-PCR.
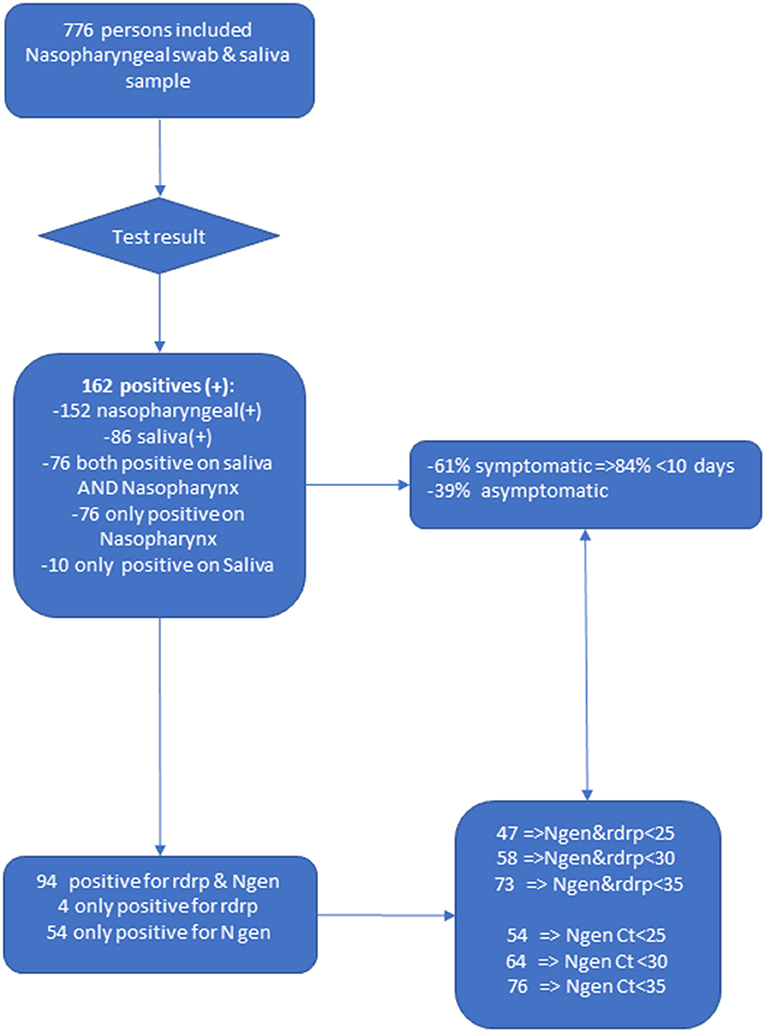
PCR testing is. 162 had a positive COVID-19 RT-PCR 61 were mildly symptomatic and 39 asymptomatic. A PCR-test from throat nasal or saliva.
These are not accepted. How They Work and What They Bring to COVID-19 Traditional COVID-19 testing is a two-part process. The Clinical Labs COVID-19 PCR Test for Travel provides travellers with a downloadable results certificate which includes the details listed below allowing you to meet travel requirements prior to boarding.
FDA EUA RT-PCR assay for the detection of SARS-CoV-2 was utilized as the comparator method for the study. Test on your location. Are serology tests accepted.
-19 Antigen Saliva Test Kit vs. The sensitivity of RT-PCR on saliva samples versus nasopharygeal swabs varied depending on the patient groups considered or on Ct thresholds. Covishield Was 63 Effective In.
One can be infected from respiratory droplets when an infected person sneezes or coughs. Ambry COVID-19 RT-PCR Test Saliva Collection Kit Use Upon receiving the kit by mail the user must read the package insert carefully and thoroughly before beginning the sample collection. September 2021 HP 7849.
With more cases of patients showing clinical symptoms of Covid-19. Is the negative COVID-19 PCR test required no more than 3 days before boarding my flight or before arrival in. Food and Drug Administration FDA has authorized the SalivaDirect PCR COVID-19 test created by the Yale School of Public Health for use with pooled saliva samples.
Doctors seek new detection norms for strains that beat RT-PCR. While results can be turned around in a few hours in urgent cases they typically take 2448 hours from when a sample is taken. A total of 32 of 58 55 patients were.
This test is approved as part of Hawaiis trusted testing partner program and for travel to Bermuda. Travellers To Pay For RT-PCR Test Wait Up To 6 Hours At Delhi Airport Amid Third Wave Scare COVID-19 Vaccine. MANILA Philippines The guidelines on the use and administration of Saliva-based Reverse Transcription Polymerase Chain Reaction RT-PCR testing.
Symptoms can range from mild or no symptoms to severe illness. RT PCR RT-PCR Total Negative COVID-19 Antigen Saliva Test Kit Positive 40 2 42 Negative 5 137 142 Total 45 139 184. The Department of Health clarifies that the use of saliva as an alternative specimen for RT-PCR testing among Philippine Red Cross PRC laboratories is not awaiting review of accuracy by the Research Institute for Tropical Medicine RITM and has already been approved as of 21 January 2021.
There were 10 62 patients with a. These are not accepted. SalivaDirect removes the extraction step replacing it with something thats really simple.
Yes this is accepted. On the Use of Saliva as Alternative Specimen for RT-PCR Testing. Paired saliva and NP samples were investigated by RT-PCR Cobas 6800 Roche-Switzerland Basel Switzerland and by two rapid antigen tests.
Herlev Hovedgade 15B 2730. If the sample contains the virus its RNA will be extracted. COVID-19 RT-PCR Saliva Test.
Accepted tests include Polymerase Chain Reaction PCR which may also be reported as RT-PCR or PCR. Results of PCR saliva-based tests are available within 48 hours after being received by the lab. 12 2021 A simplified COVID-19 testing protocol can detect minimal quantities of the SARS-CoV-2 using samples from the nose and throat as well as saliva and may be useful in testing.
Pooled testing allows labs to combine saliva samples from multiple individuals into a single tube and process the batch as a single test. The two DNA template strands are then separated. This approach maintains the.
Saliva testing for COVID-19 uses the same laboratory test to confirm a case as the nasopharyngeal swab reverse transcription polymerase chain reaction RT PCR. Coronavirus COVID-19 is an illness caused by a virus that is transmitted from person to person. Samples that can be collected for this test include.
834891 Fig 3 versus 920 from nasopharyngeal swabs 95CI. Oronasopharyngeal swabbing can be discomfortable to the patients requires trained healthcare personne. Expedited processing is also available.
The saliva test results taken via a Zoom call are available within 12 to 48 hours upon being received by the lab. The testing process begins when healthcare workers collect samples using a nasal swab or saliva tube. PCR testing takes several hours and is not considered a rapid test.
The overall sensitivity of the RT-PCR test from saliva samples was 865 95CI. The SARS-CoV-2 virus which is the pathogen that causes COVID-19 uses RNA as its genetic material. We enrolled 776 persons at various field-testing sites and collected nasopharyngeal and pooled saliva samples.

Saliva Tests How They Work And What They Bring To Covid 19 The Scientist Magazine
Frontiers Prospective Comparison Of Saliva And Nasopharyngeal Swab Sampling For Mass Screening For Covid 19 Medicine

A Nasal Spray To Protect From Covid The Week S Top Science Stories World Economic Forum
How Coronavirus Throat Nose Tests Work Rt Pcr Method Explained
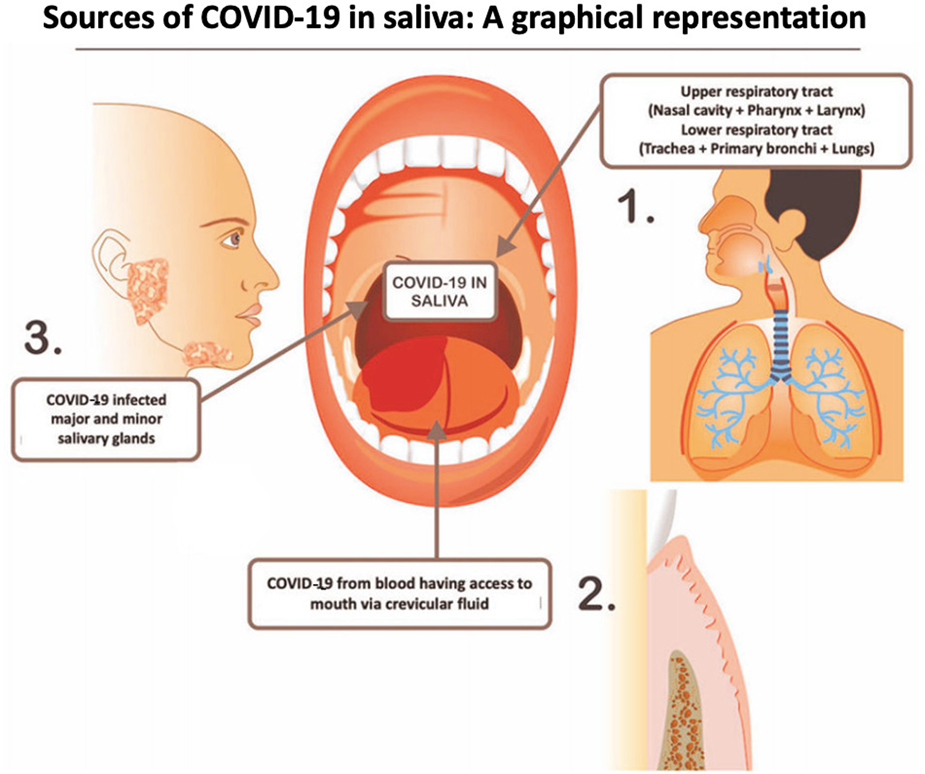
Exploring Salivary Diagnostics In Covid 19 A Scoping Review And Research Suggestions Bdj Open

Tidak ada komentar:
Posting Komentar